Doctors, Device Makers: Close Ties
A lawsuit involving health-products company Johnson & Johnson JNJ+0.59% offers a glimpse into the close ties between the medical-device industry and doctors who provide or can influence the information consumers hear about medical products.
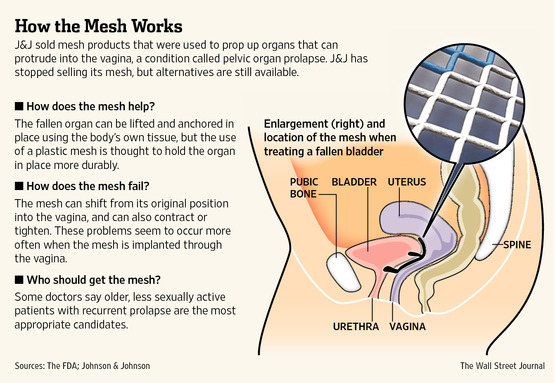
At issue is a long-running debate over the company’s transvaginal mesh products, used to surgically repair a condition called pelvic organ prolapse. The kits are currently the subject of some 5,000 product-liability lawsuits against J&J’s Ethicon division in New Jersey and 15,000 in federal court, according to plaintiffs attorney Adam Slater, who is the co-liaison counsel for the thousands of cases in New Jersey. Other manufacturers of similar products also are being sued.
J&J said it is “confident the evidence shows that Ethicon acted appropriately and responsibly in the research, development and marketing of our pelvic mesh products, and will continue to vigorously defend all product-liability lawsuits concerning their use.”
Emails and other documents related to the Ethicon suits, provided by the company during the discovery process, suggest that a physician paid by J&J as a consultant sought to influence the language in treatment guidelines for prolapse put forth by a medical society in 2007. The company also sought to change the language of a high-profile research paper on the mesh procedure published in the New England Journal of Medicine in 2011, the documents show.
The editors of NEJM currently are fighting a subpoena to testify in some of the Ethicon lawsuits. The hearing is scheduled for Friday. NEJM declined to comment on the subpoena or litigation.
Financial relationships between the medical community and device manufacturers are commonplace and legal. Companies and doctors say the relationships are important to improve medical education and inform doctors about the creation of new or improved products. Top doctors in a field often take on consulting roles to companies for which they are paid tens of thousands of dollars or more. Efforts like the 2010 Physician Payment Sunshine Act have been implemented to encourage transparency about the relationship between doctors and the industry.
There have been concerns about conflicts of interest that could arise between physicians and the industry. Payments that constitute kickbacks from companies have sparked regulatory and legal actions. But the mesh documents suggest that potential influence over medical practice also can be more subtle.
“Ethicon’s relationships with the medical community concerning our pelvic mesh products have been appropriate and responsible,” J&J said. “As part of our efforts to advance the standard of care for women’s health, it is customary for us to have discussions with leading physicians and researchers.”
The use of transvaginal mesh was a popular alternative to more-invasive surgery to prop up organs that can protrude into the vagina, causing discomfort. In 2010, according to the FDA, 75,000 women had the transvaginal mesh procedure. But some women have reported cases where the mesh shrinks or erodes from its original placement, sometimes causing significant pain.
Johnson & Johnson removed its kits from the market in 2012, saying the move was made for business reasons.
Back in 2007, however, J&J was still trying to expand sales. The American College of Obstetricians and Gynecologists was about to publish practice guidelines calling the mesh kits experimental. Company executives sought input from one of its physician consultants, Vincent Lucente, a well-known urogynecologist and advocate of the procedure, according to emails in court records.
On Feb. 6, Dr. Lucente, based in Allentown, Pa., told J&J executives that the guidelines’ characterization of the product as experimental needed to be changed, according to the emails, because the devices had been cleared by the FDA and the language would scare off patients, he explained later in an interview. Other J&J consultants concurred, according to emails.
David Robinson, then medical director of Ethicon, the division of J&J that marketed the mesh products, agreed that the word was a problem but said that J&J couldn’t be overtly involved in lobbying to change the wording, the emails show. “This is not an issue that we can be out in the forefront on as it will only appear too self-serving,” Dr. Robinson responded that same day. “Our work remains in the background.”
ACOG’s initial bulletin was published in February 2007 and stated: “Given the limited data and frequent changes in the marketed products…the procedures should be considered experimental and patients should consent to surgery with that understanding.”
Shortly after, the group began receiving emails, letters and phone calls from members who objected to the use of the word “experimental,” according to ACOG. J&J denies that it was involved in any of these efforts.
Dr. Lucente said in a recent phone interview that he called one of the leaders of ACOG to explain his concerns after the bulletin came out.
In late August, Dr. Lucente alerted Price St. Hilaire, Ethicon’s U.S. group marketing director, that ACOG was changing its practice bulletin, according to emails. “Note, no further use of the word experimental!” wrote Dr. Lucente. “Well, this is one I’m taking credit for. I led the charge on this and never thought we would get a complete replacement of the earlier bulletin.”
Dr. Lucente said he was paid about $800,000 by Ethicon as a consultant over a 10-year period to educate other doctors. J&J said he was paid to tell doctors about the safety and efficacy of Ethicon medical devices.
Some experts contended that the organization succumbed to commercial pressures to change the guidelines.
The new guidelines with the word “experimental” removed came seven months after the first bulletin. The speed of the turnaround was “unprecedented” and seemed related to concerns that insurers wouldn’t reimburse doctors for devices or procedures deemed experimental, according to L. Lewis Wall, a urogynecologist and professor at Washington University who published an opinion piece criticizing ACOG for the change. ACOG says that it usually reviews its guidelines every 18 to 24 months.
ACOG says it clarified the wording in the bulletin in response to members who complained that the meaning of “experimental” was ambiguous.
One of the authors of the original practice guidelines, Anne Weber, called the purported rationale for changing the wording “disingenuous at best.”
“Most of the clinicians who objected to the use of the word ‘experimental’ understood only too well exactly what meaning was intended,” wrote Dr. Weber, an obstetrician-gynecologist in Pittsburgh, in a letter to the editor published in the International Urogynecology Journal in 2009. “Such clinicians were concerned that insurance companies would not cover procedures labeled experimental.”
J&J said that the clarification was widely discussed in the medical community. “Ethicon did discuss the issue with our consultants,” but Ethicon wasn’t asked or directed to take a position, J&J spokesman Matthew Johnson said. J&J declined further comment.
ACOG, for its part, said that it doesn’t know whether Dr. Lucente was one of the members who responded to the guidelines. The group said it wouldn’t have known about his relationship with the industry because it requires financial disclosures only when members serve in leadership roles, which Dr. Lucente didn’t in 2007.
Companies also may exert influence in the medical community through research papers. In a clinical trial of a transvaginal mesh procedure conducted by Swedish researcher Daniel Altman, a professor at the Karolinska Institute, J&J gave $750,000 to the university, along with grants from the Swedish government, to support research that Dr. Altman initiated.
Dr. Altman said in an interview that he fought from the beginning to conduct the trial independently from the company. The original contract stipulated that J&J would own the data and could stop the trial whenever they wanted, so Dr. Altman said he refused to sign it. Ultimately, the contract gave Dr. Altman full control over the trial.
When the manuscript was published in the New England Journal of Medicine in 2010, the disclosure statement said that the company hadn’t been involved in the design of the study, the data collection or analysis, or in the writing of the paper.
Emails and depositions in court documents, however, show that company executives combed through a draft of the report before publication and gave Dr. Altman detailed feedback.
One exchange depicts a back-and-forth about how to best write about one of the adverse events, sexual pain. On Aug. 20, 2010, Piet Hinoul, the world-wide medical affairs director for women’s health and urology of Ethicon, suggests that Dr. Altman remove the number of women who experienced pain during sexual intercourse and instead to report overall sexual satisfaction scores, which showed no significant difference between the group that received the mesh procedure compared with a standard surgery, according to court documents.
“We feel that selecting one reported outcome somehow will be used by the mesh antagonists, whilst you may just as well have selected overall sexual satisfaction to go in the manuscript which would show a completely different impression,” wrote Dr. Hinoul in an email to Dr. Altman and several J&J colleagues.
The abstract of the final paper doesn’t mention sexual pain or satisfaction but includes the percentage of women who needed additional surgery to correct mesh exposure.
Dr. Altman said in an interview he recalled using very little of the J&J executives’ suggestions and most of the changes were linguistic, because he isn’t a native English speaker.
“As is customary for independent, investigator-initiated research studies that receive funding from a manufacturer, Ethicon provided commentary on a draft of the Altman study after Dr. Altman asked for our comments,” said J&J in a statement. “Ethicon did not inappropriately seek to influence the study results or the author’s conclusions. We did not know what, if any, changes Dr. Altman would make until we saw the final paper.”
In 2013, the NEJM issued a correction acknowledging the company reviewed the study protocol and a draft of the manuscript but continues to state it had no involvement in data collection and analysis or the decision to submit the results for publication. A spokeswoman for NEJM said, “The responsibility always lies with the author to disclose the relevant information.”
Dr. Altman said he interpreted the meaning of whether the company had “involvement” in the study to mean who had responsibility for the data and the paper.
“We really tried to be open and honest,” said Dr. Altman.
Write to Shirley S. Wang at shirley.wang@wsj.com


